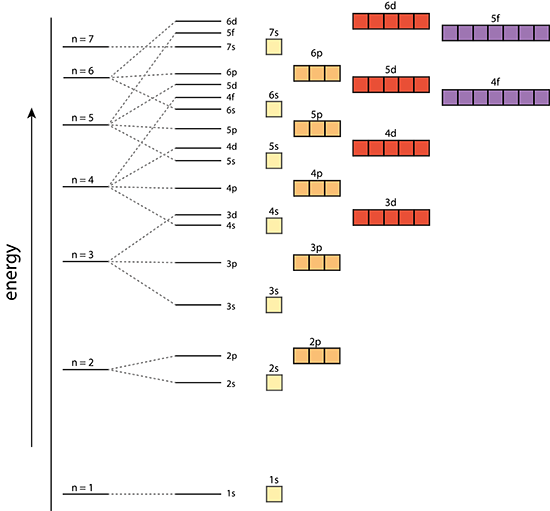
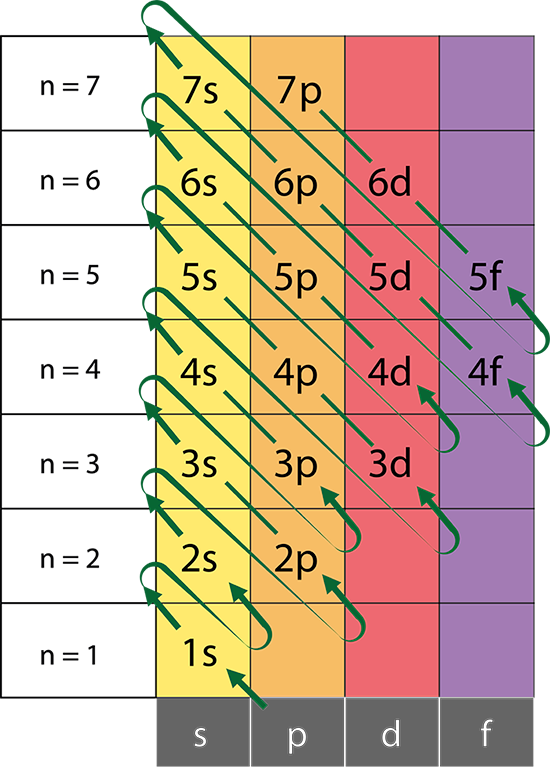
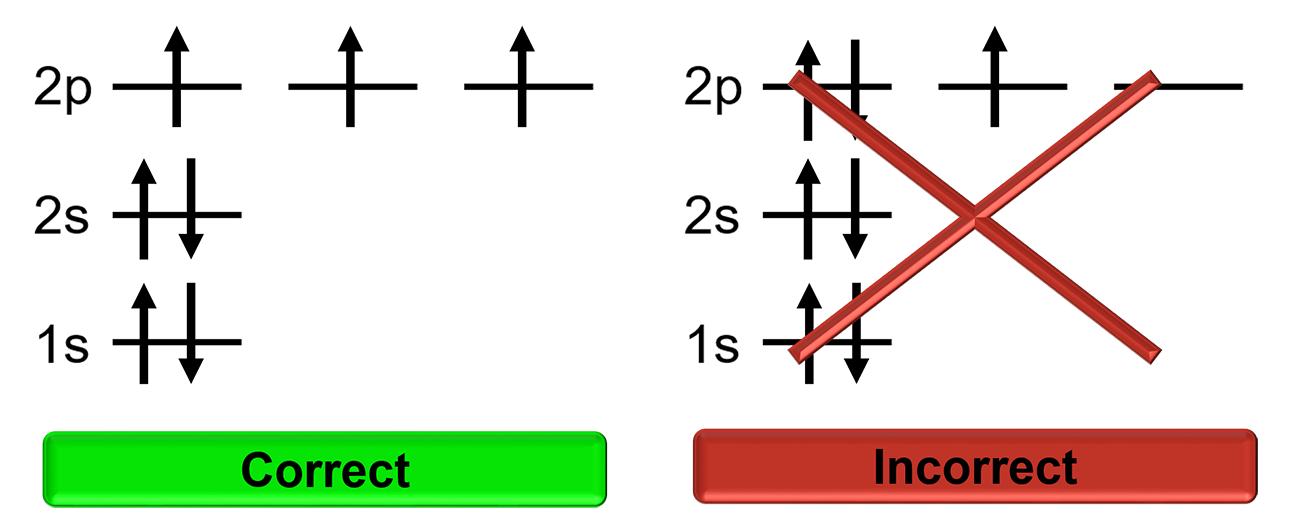
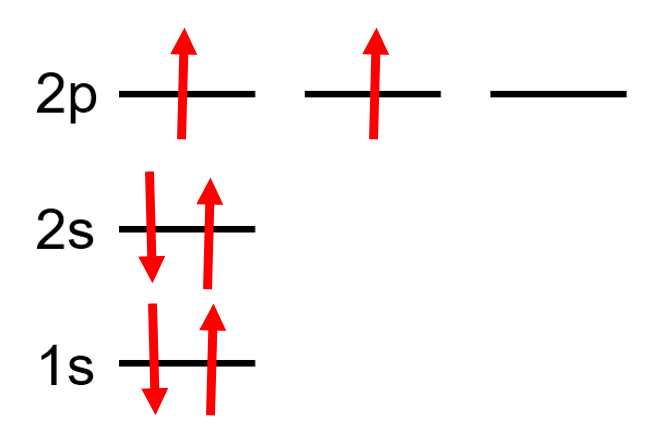
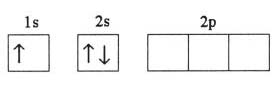
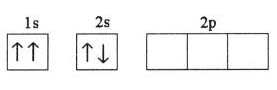
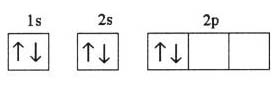
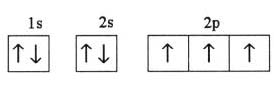
What is the problem asking?
Atomic mass of silicon = u = ?
Apply your Knowledge
Learn the theory at your own pace, try examples and test your new abilities with the quiz.
(Click the banner to open.)
The Atom Theory
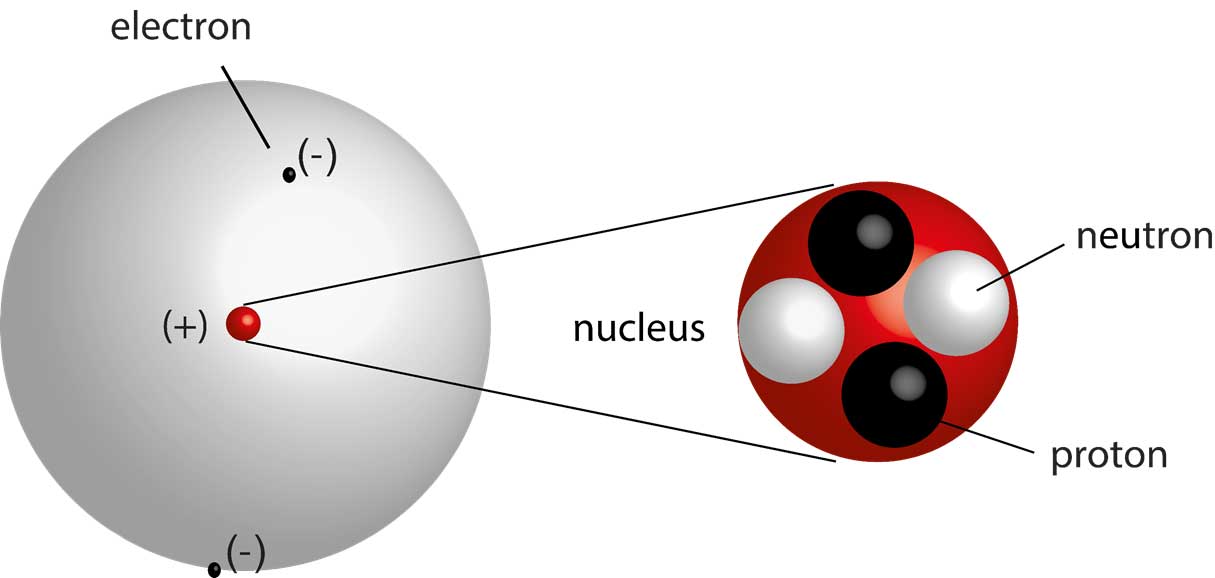
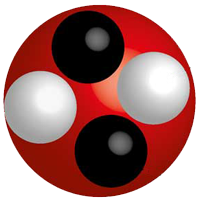
Nucleus
The small dense core of an atom consisting of neutrons and protons. Neutrons and protons have approximately the same mass.

Neutron
A neutral particle located in the nucleus of an atom.

Proton
A positively charged particle located in the nucleus of an atom.

Electron
A negatively charged particle located in the volume of space surrounding the nucleus of an atom.
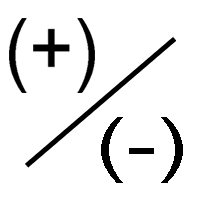
Charge
Electrons and protons have charge that is equal and opposite, because of this we can say the relative charge in atomic units is one.
A neutral atom has the same number of protons as electrons.
| Particle | Charge (Coulombs) | Mass (kilograms) | Relative Charge | Mass (unified atomic mass units) |
|---|---|---|---|---|
| Proton | \(+1.6\) x \(10^{-19}\,C\) | \(1.673\) x \(10^{-27}\,kg\) | +1 | 1.0073 \(\,u\) |
| Neutron | No Charge | \(1.675\) x \(10^{-27}\,kg\) | 0 | 1.0087 \(\,u\) |
| Electron | \(-1.6\) x \(10^{-19}\,C\) | \(9.109\) x \(10^{-31}\,kg\) | -1 | \(5.4858\) x \(10^{-4}\,u\) |
Unified atomic mass units (u):
\(\frac{1}{12}\) the mass of a carbon atom containing six protons and six neutrons.
Using this measurement, the mass of a proton and neutron is approximately \(1 \,u\).
The masses of all elements are defined using unified atomic mass units.
\(1\,u = 1.661\)x\(10^{-27}\,kg = \frac{1}{12}\) the mass of a carbon atom
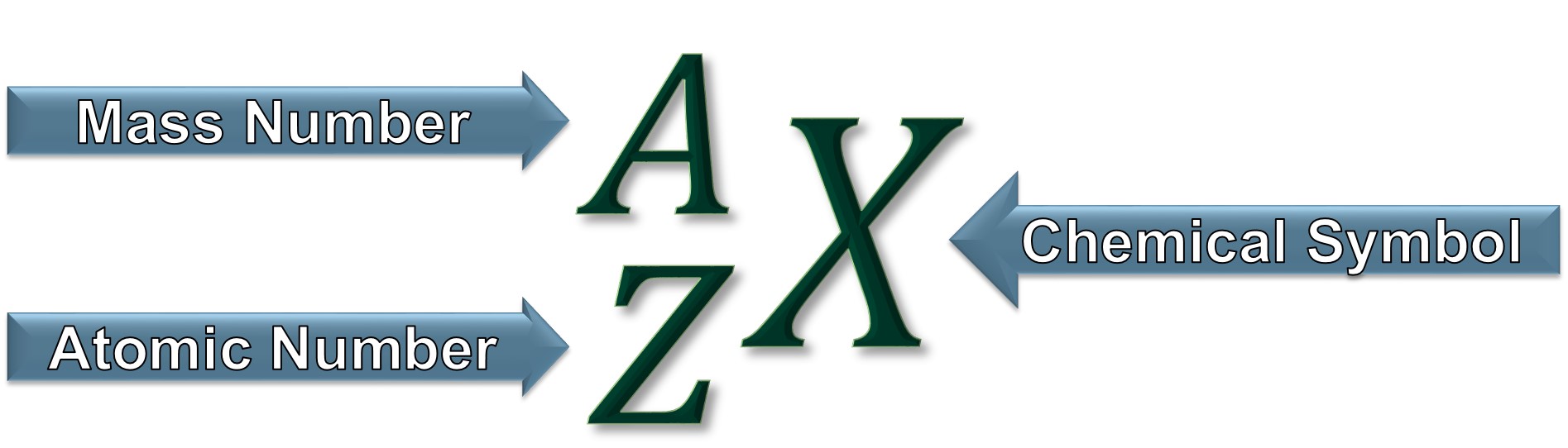
Z = number of protons = atomic number
A = number of protons + number of neutrons = mass number
Element
One or more atoms of the same type.
Chemical Symbol (X)
A one or two letter abbreviation assigned to each element.
Atomic Number (Z)
The number of protons in the nucleus of an atom. The type of atom/element is determined by the number of protons.
Mass Number (A)
The sum of the number of neutrons and protons in the nucleus of an atom.
The number of protons defines the type of atom or element!
Isotopes are atoms of the same type that have the same number of protons and varying numbers of neutrons.
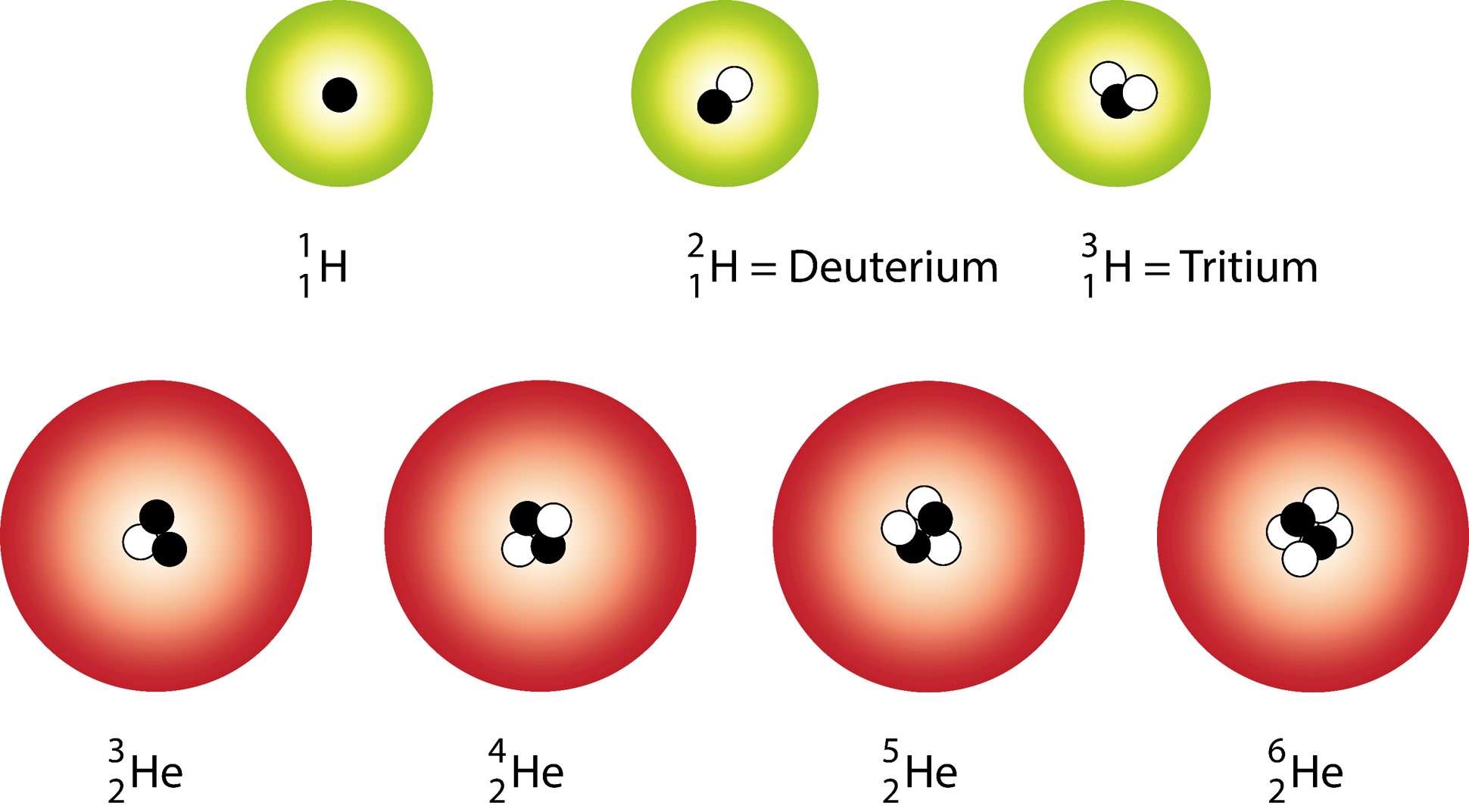
We know:
- The number of protons determines the type of atom or element.
- Neutrons and protons comprise the nucleus of an atom and have the same approximate mass.
Isotopes:
- The number of protons determines the type of atom or element.
- Isotopes of elements are distinguished by the mass number (A).
Isotopes Example:
Atoms of an element that have a different number of neutrons.

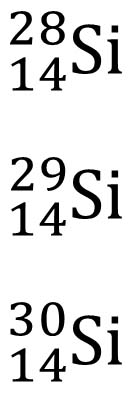
28-14=14 neutrons
29-14=15 neutrons
30-14=16 neutrons
Isotope Notation
Including the atomic number is redundant when representing isotopes. It is common to use the following notation: X-A.
For example: Si-28, Si-29, Si-30
We know:
- Isotopes are atoms of the same type that have a different number of neutrons.
- The mass of an atom is dictated by the mass of protons and neutrons because the mass of an electron is negligible in comparison.
Atomic Mass:
- Is the weighted average of isotopic masses based on the natural abundance of each isotope.
- The natural abundance is the relative amount of each isotope found in a natural sample of any element. Natural abundance is relatively constant and unique for each element.
The atomic mass of an element as seen on the periodic table is a weighted average of isotopic masses of that element.
Atomic Mass Example:
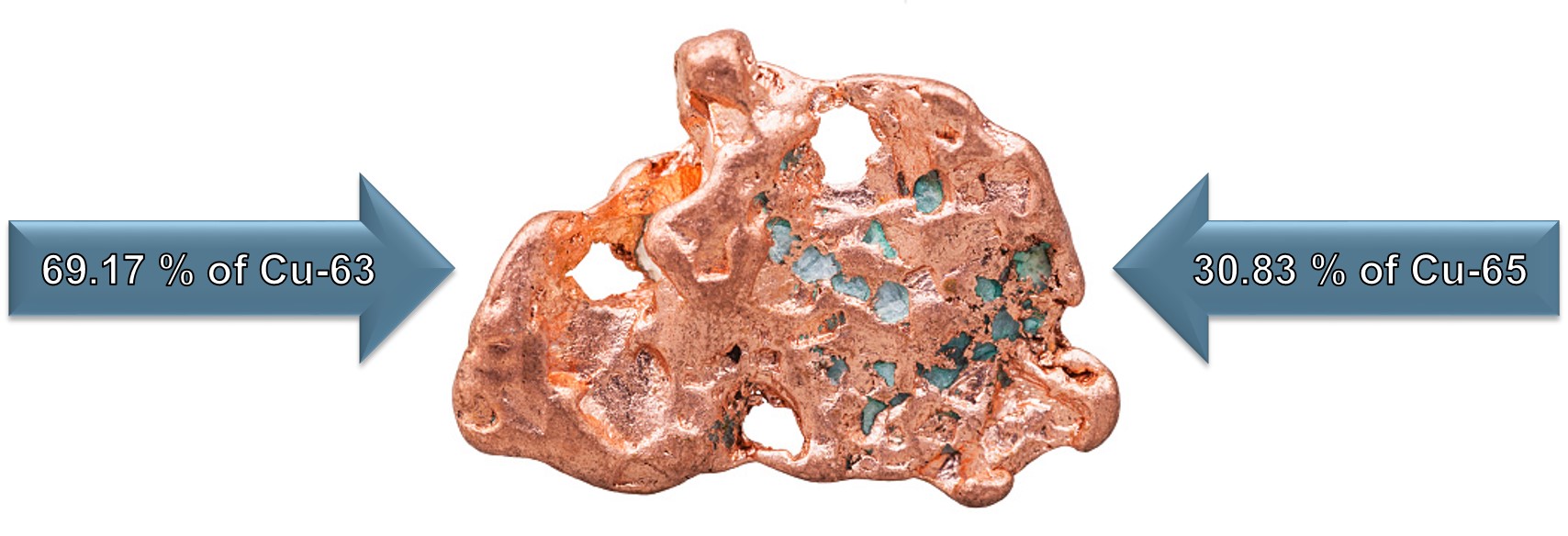
This Copper (Cu) sample will contain approximately 30.83 % of Cu-65 and 69.17 % of Cu-63
Atomic Mass Calculations:
Atomic mass for an element can be calculated with the following formula:
$$\sum_{n} \text{(fraction of isotope n) x (mass of isotope n)}$$
In simple terms this formula is telling us that atomic mass can be calculated by:
1) Multiplying each isotopic mass of an element by its relative abundance.
2) Adding the resulting masses of the isotopes together.
An atomic mass calculation is included in the example section!
A measurement similar to a pair, dozen or score. A pair is 2 objects, a dozen is 12 objects and a score is 20 objects.
1 mole \(= 6.022\)x\(10^{23}\) objects = Avogadro's number

One mole of people is the Earth’s population multiplied by \(9\)x\(10^{13}\).

One mole of bricks is equivalent to \(2\)x\(10^{14}\) Great Walls of China.

One mole of sand is the entire Sahara Desert!
Defining the Mole:
The mole is defined using the C-12 isotope.
One mole = number of atoms in exactly 12 g C-12.
12 g of C-12 = 1 mole of C-12 atoms = exactly \(6.02\)x\(10^{23}\) C-12 atoms
The mass of one mole of C-12 atoms is 12 g

1 mole of carbon (C) = \(6.02\)x\(10^{23}\) atoms
Molar Mass:
Using C-12 as a standard for the mole gives a relationship between mass, number of atoms, and unified atomic mass units.
The mole can be used to count atoms based on their mass.
The molar mass of an element is the mass of one mole of each element.

1 mole of carbon (C) \(6.02\)x\(10^{23}\) atoms

1 mole of sulfur (S) \(6.02\)x\(10^{23}\) atoms

1 mole of gold (Au) \(6.02\)x\(10^{23}\) atoms
Molar Mass of an Element:
The molar mass of an element is the mass of one mole of each element.
Molar mass
the mass of the
x
atoms of each element.
Molar mass is numerically equivalent to an element’s mass in unified atomic mass units
.
Molar Mass \(\frac{\text{g}}{\text{mol}}\) is numerically equivalent to atomic mass \(u\)

Metals, Non-Metals and Metalloids
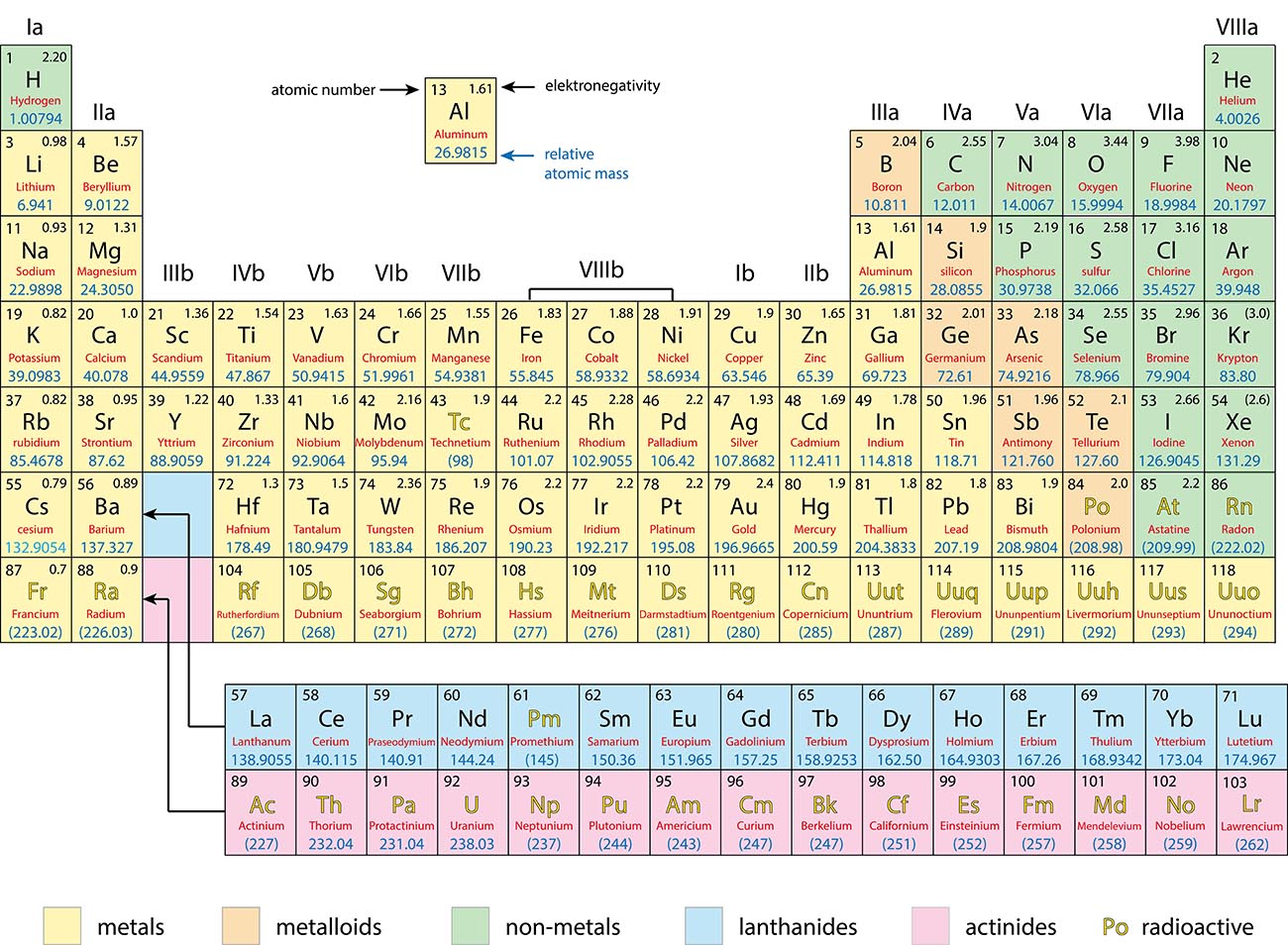
Periodic Trends
Metals:
Generally good conductors of heat and electricity
Tend to lose electrons in a chemical reaction
Non-Metals:
Generally poor conductors of heat and electricity
Tend to gain electrons in a chemical reaction
Metalloids:
Have properties of both metals and non-metals.
Main Group Elements and Transition Metals
2.jpg)
Periodic Trends
Main Group:
Elements that have predictable properties
Transition Metals:
Elements that have unpredictable properties.
Lanthanoids and Actinoids:
Elements that are placed separately for a more compact periodic table. They have similar properties to the elements lanthanum and actinium.
Groups and Periods of the Periodic Table
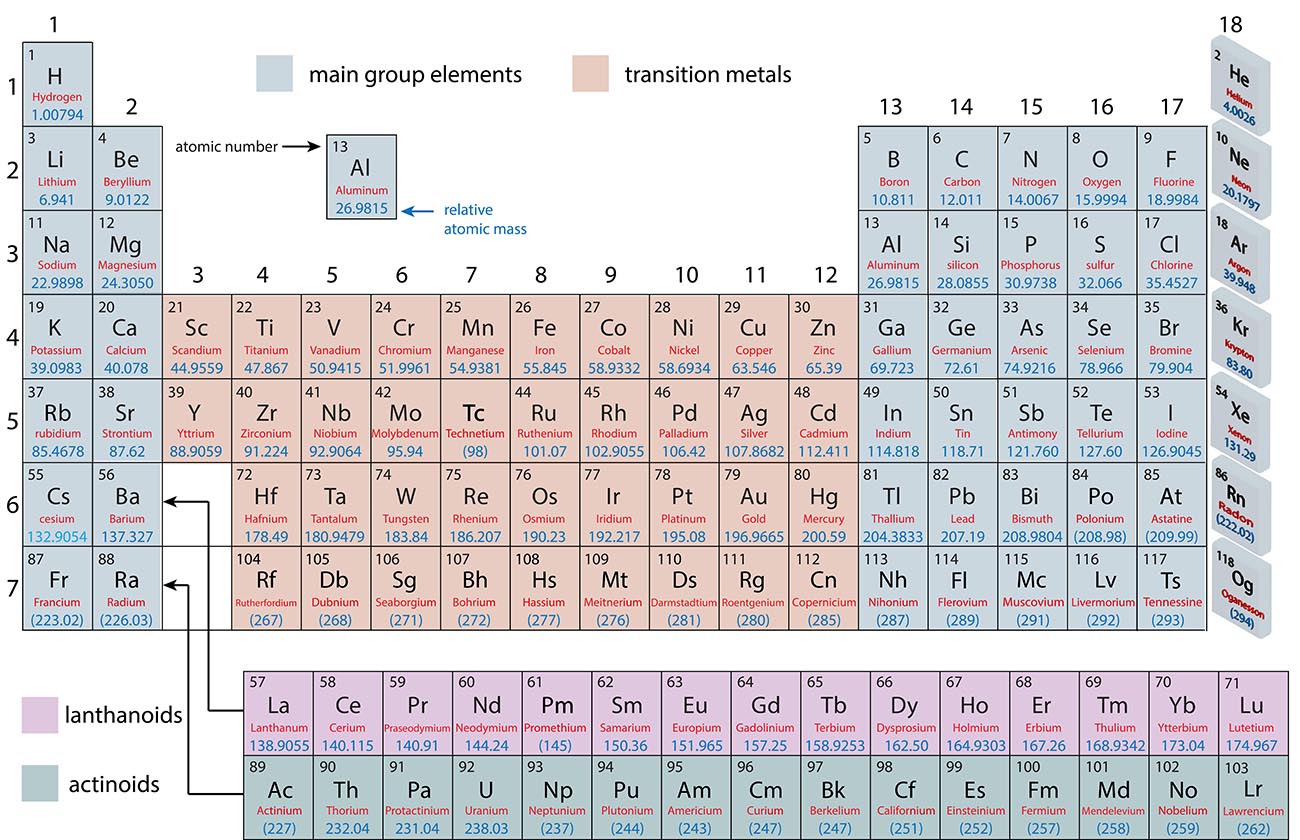
Periodic Trends
Each column in the periodic table is called a group and numbered 1-18
Each row in the periodic table is called a row and numbered 1-7
The elements in a group have similar properties.
For example: The noble gases in group 18 are all relatively unreactive.
Common Main Group Ions:
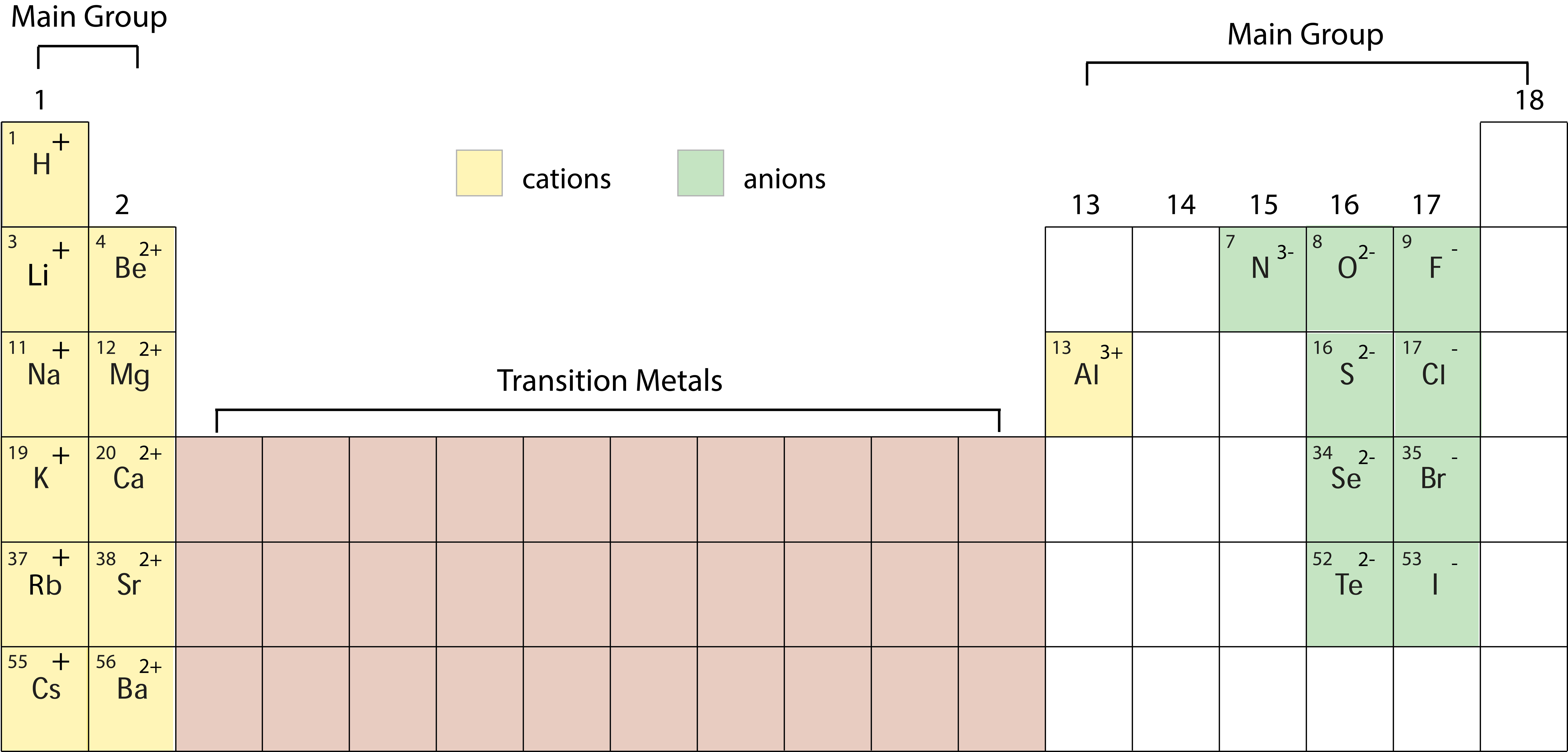
We know:
- The number of protons determines the type of atom or element.
- Neutrons and protons comprise the nucleus of an atom, and have the same approximate mass.
Ions:
- One or more atoms that has gained or lost an electron(s).
- Ions are charged particles.
- The periodic table can help predict which ions will form.
Ions and Groups:
- A main group non-metal will generally gain electrons.
- A main group metal will generally lose electrons.
Ions are charged particles that have gained or lost an electron(s).
Cation (+):
One or more atoms that has lost an electron(s).
A main group METAL will LOSE electrons to form a CATION with the same number of electrons as the nearest noble gas.
Anion (-):
One or more atoms that has gained an electron(s).
A main group NONMETAL will GAIN electrons to form an ANION with the same number of electrons as the nearest noble gas.
Ion Notation:
Ions are represented as the chemical symbol of the ion with the charge of the ion noted as a superscript to the right.
\(\text{X}^{\text{charge}}\)
Lithium ion \(=\text{Li}^{\text{+}}\), Oxygen ion \(=\text{O}^{2-}\)



.png)