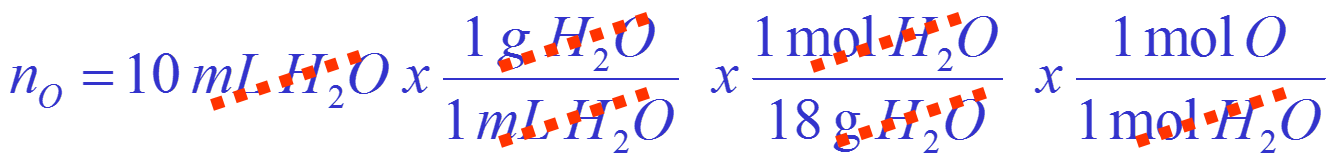What is the problem asking?
The volume of debris released = \(m^{3}\) = ?
Apply your Knowledge
Learn the theory at your own pace, try examples and test your new abilities with the quiz.
(Click the banner to open.)
Chemical Problem Solving Strategy Theory
A 'book-keeping' method for units in a calculation
Unit analysis helps avoid errors in a multi-step calculation and provides the units for the final answer.
Over all method of “unit analysis”:
1) Write the units with every number you include in a series of calculations
2) String your calculations together as a series of multiplications or divisions before doing any math
3) Cancel your units to see the calculation evolve
* Gives you a hint about the next step *
Calculations: Converting from One Unit to Another
Unit analysis:
A method that uses a conversion factor to convert a quantity expressed in one unit to an equivalent quantity in a different unit.
Conversion factor:
States the relationship between two different units.
original quantity x conversion factor = equivalent quantity
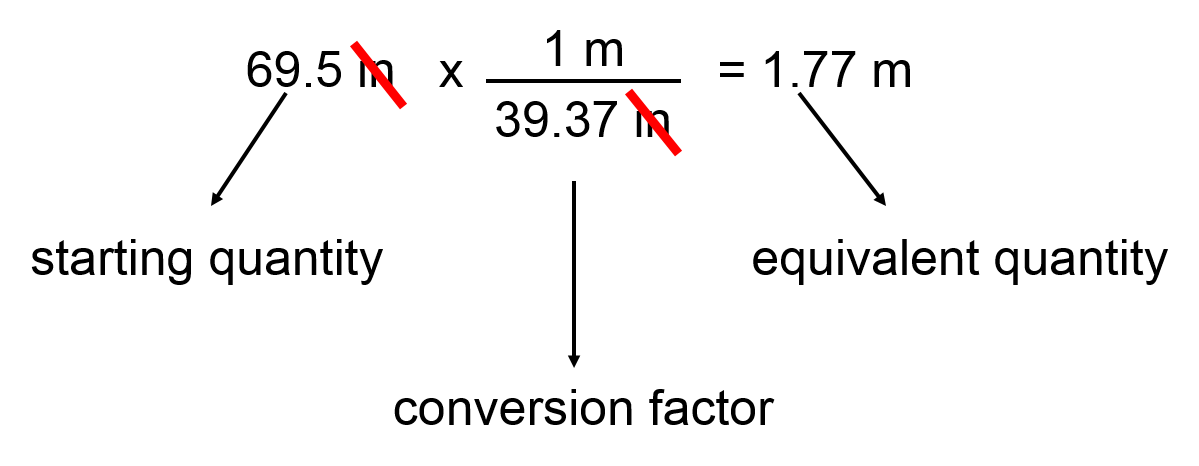
For example converting between length units
Given that 1 meter = 39.37 inches
Conversion factors \(\frac{1\,m}{39.37 \,\text{inches}} \,\text{or} \,\frac{39.37 \,\text{inches}}{1\,m}\)
The same relationship, just invert as necessary to give you the units you need!
So to take a simple example, if we needed to convert 69.5 inches into meters we could do the following
FACT:
The more you use the “long method” of converting units, the fewer errors you will make!
How many moles of oxygen atoms are there in a 10 mL volume of water?
What is being asked?
Given a volume can you calculate a number of atoms?
What data is provided?
Data: 10 mL of water
What do I need to know?
Need to know: water is \(H_{2}O\), density of water, molecular weight of water
How do I need to state the answer?
Answer in moles of oxygen O
Convert volume of water to moles of oxygen
Calculation is: Volume of \(H_{2}O\) \(\to\) mass of \(H_{2}O\) \(\to\) mols of \(H_{2}O\) \(\to\) mols of O